-
Cation Size and Anion Anisotropy in Structural Chemistry of Metal Borohydrides. The Peculiar Pressure Evolution of RbBH4

Y. Filinchuk, A.V. Talyzin, H. Hagemann, V. Dmitriev, D. Chernyshov and B. Sundqvist
Inorganic Chemistry, 49 (11) (2010), p5285-5292


DOI:10.1021/ic100359v | unige:14769 | Abstract | Article HTML | Article PDF
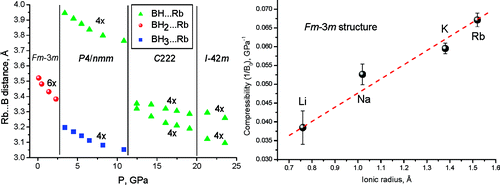
The pressure evolution of RbBH4 has been characterized by synchrotron powder X-ray diffraction and Raman spectroscopy up to 23 GPa. Diffraction experiments at ambient temperature reveal three phase transitions, at 3.0, 10.4, and 18 GPa (at 2.6, 7.8, and ~20 GPa from Raman data), at which the space group symmetry changes in the order Fm-3m(Z=4) → P4/nmm(2) → C222(2) → I-42m(4). Crystal structures and equations of state are reported for all four phases. The three high-pressure structure types are new in the crystal chemistry of borohydrides. RbBH4 polymorphs reveal high coordination numbers (CNs) for cation and anion sites, increasing with pressure from 6 to 8, via an intermediate 4 + 4 coordination. Different arrangements of the tetrahedral BH4 group in the Rb environment define the crystal symmetries of the RbBH4 polymorphs. The structural evolution in the MBH4 series is determined by the cation’s size, as it differs drastically for M = Li (CNs = 4, 6), Na (CN = 6), and Rb. The only structure common to the whole MBH4 family is the cubic one. Its bulk modulus linearly decreases as the ionic radius of M increases, indicating that the compressibility of the material is mainly determined by the repulsive BH4···BH4 interactions.